Your Partner for CDSCO Medical Device Registration, Licensing, and Market Access
Bringing a medical device to the Indian market today means staying ahead of more than just paperwork, it means responding to real-time CDSCO updates, adapting to expanding device notifications, and understanding technical documentation that changes with each class and format. Whether you're registering a diagnostic kit, a connected wearable, an orthopedic implant, a drug eluting stent or a digital SaMD , India’s regulatory journey demands more than a checklist. At DDReg, we provide the clarity, speed, and local authority alignment you need to move from application to approval without losing time or traction.
At DDReg, we provide strategic, end-to-end regulatory support from device classification and predicate device identification in India, to securing your medical device import license, filing through SUGAM , and supporting your lifecycle obligations post-approval..
India’s Medical Device Regulatory Framework
India regulates medical devices under the Medical Device Rules (MDR), 2017, enforced by CDSCO, with updates released via notifications and guidance documents. Devices are categorized based on risk class as follows:
| Class | Risk Level | Examples | Approval Body |
|---|---|---|---|
| A | Low Risk | Thermometers, bandages | State Licensing Authority (SLA) |
| B | Low-Moderate Risk | Surgical gloves, infusion sets | SLA |
| C | Moderate-High Risk | IVD analyzers, contact lenses | CDSCO |
| D | High Risk | Cardiac stents, implants, SaMD for critical care | CDSCO |
All notified devices, whether imported, manufactured, or distributed—must undergo medical device product registration before entering the Indian market.
Approval Process of Class A, B, C & D Medical Devices in India
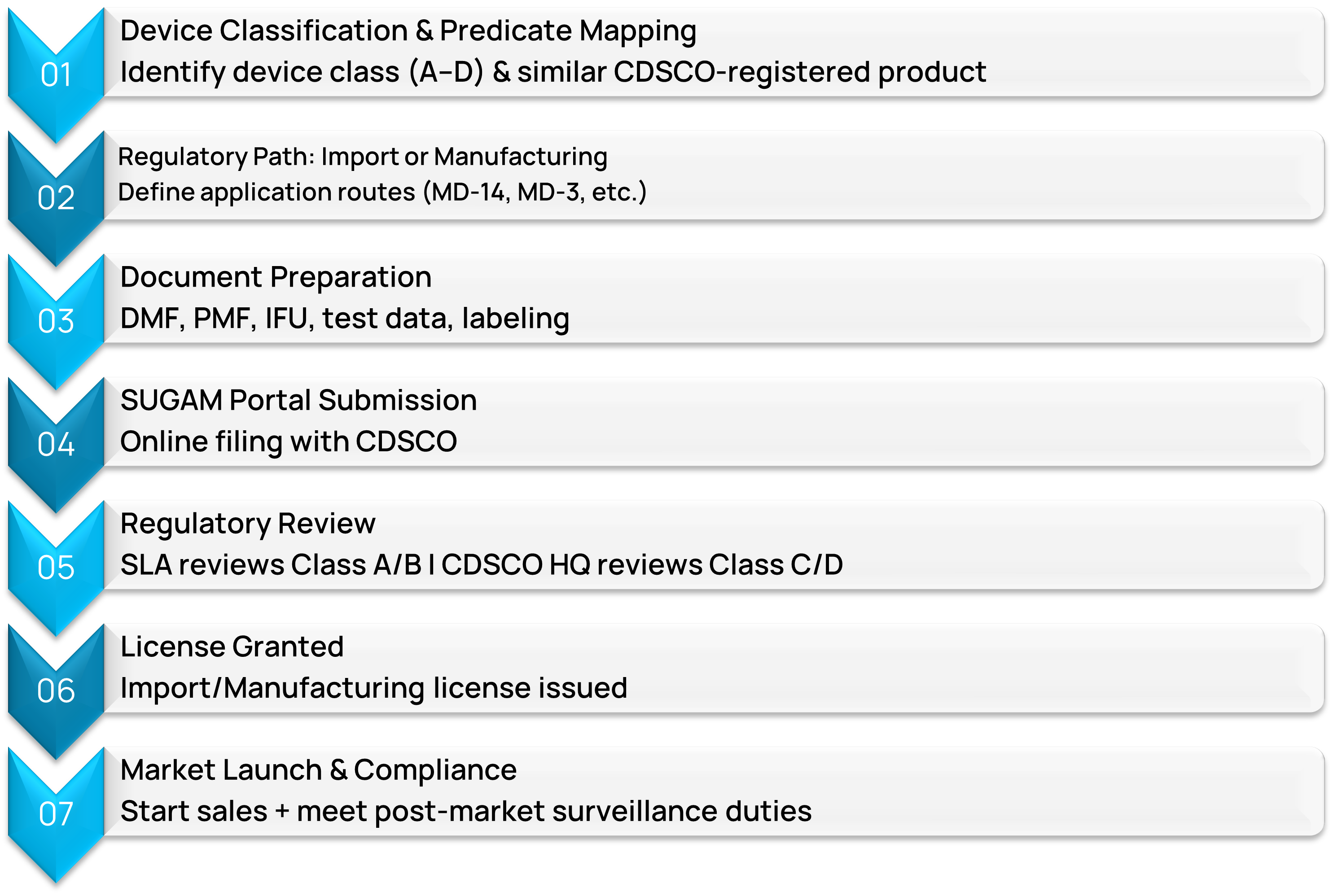
DDReg’s CDSCO Medical Device Regulatory Services
-
Device Classification & Regulatory Strategy
-
CDSCO Medical Device Registration & Import Licensing