FSSAI Regulatory Services for Food Supplements & Nutraceuticals
Bringing food supplements, nutraceuticals, or dietary products to the Indian market involves navigating a highly regulated, ingredient-specific, and claim-controlled ecosystem governed by the Food Safety and Standards Authority of India (FSSAI).
Unlike voluntary certifications, FSSAI licensing is statutorily mandated under the Food Safety and Standards Act, 2006. Whether you're a health supplement importer in India, a contract manufacturer, or a nutraceutical R&D innovator, FSSAI compliance is not just about product registration, it’s about ensuring each ingredient, label, and functional claim withstands regulatory scrutiny.
At DDReg, Food Regulatory services experts combine scientific acumen with procedural mastery to guide your product from ingredient-level evaluation to FoSCoS-based licensing, novel food clearance, and market launch, all while maintaining complete alignment with FSSAI’s evolving framework.
Key Regulatory Foundations for Health & Functional Foods in India
- Governed by Food Safety and Standards (Health Supplements, Nutraceuticals, Food for Special Dietary Use, Food for Special Medical Purpose, Functional Foods and Novel Foods) Regulations, 2016, and its amendments
- All manufacturers, marketers, or importers must hold a valid FSSAI license or registration, obtained through the FoSCoS portal
- Products must only include permitted ingredients, additives, and nutrient limits defined under Schedule VI.
- Functional claims must align with regulatory allowances; therapeutic or curative claims are strictly prohibited.
- Products outside permitted schedules require Proprietary or Novel Food approval.
Food Regulatory Pathway: FSSAI Licensing & Approval Flow
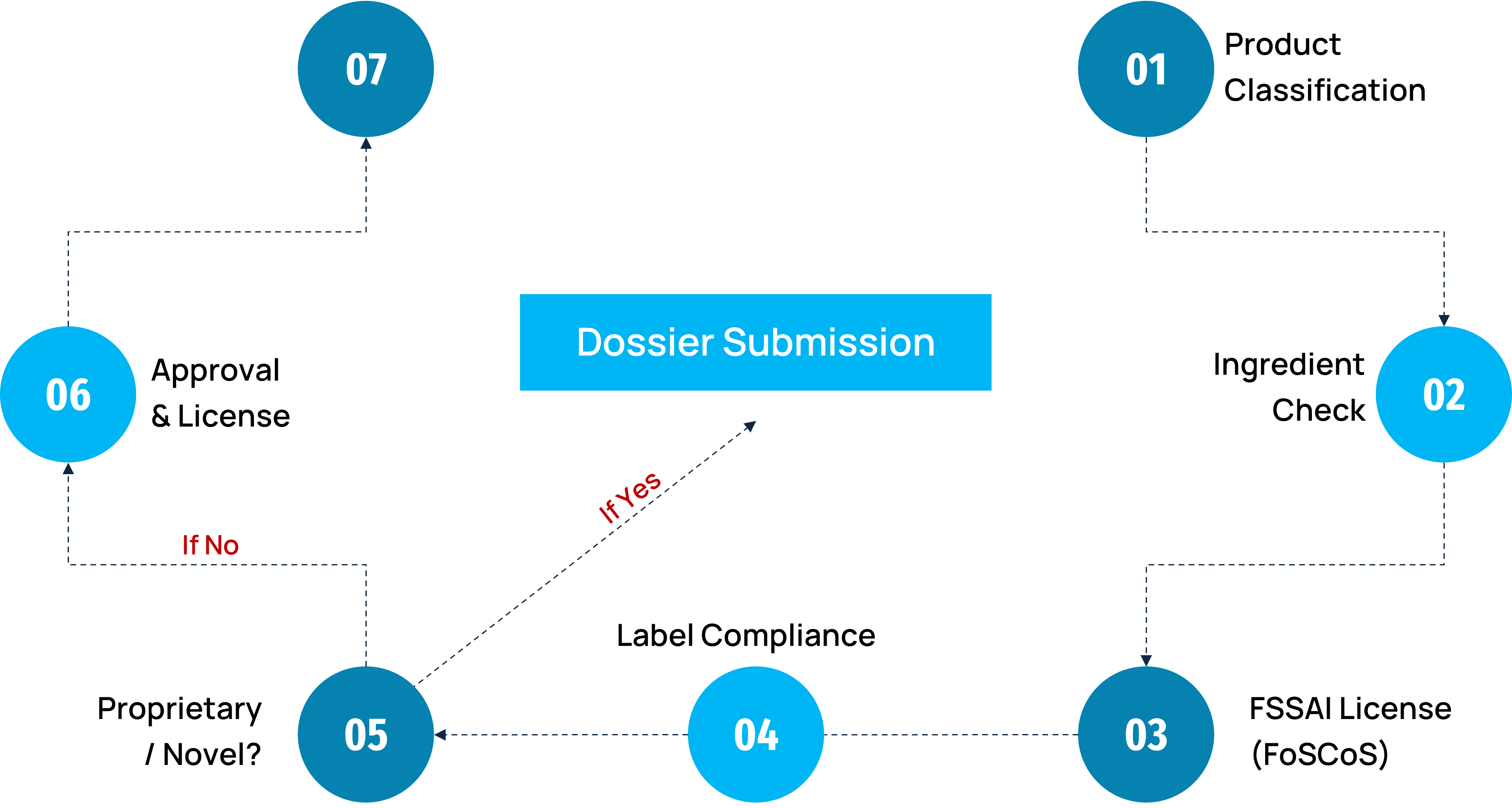
DDReg’s Regulatory Services for Food Supplements in India
-
Product Classification & Regulatory Mapping
-
FSSAI License Application (FoSCoS)
-
Label & Claims Compliance
-
Importer Support & FSSAI Clearance
-
Proprietary Product and Novel Food Approvals
-
Other related Approvals
Specialized Food Regulatory Support for Foreign Manufacturers
India’s import ecosystem for health supplements is strict, especially around ingredient permissibility, RDA limits, and label declarations. DDReg helps foreign manufacturers achieve:
- Product and label adaptation for Indian regulatory compliance
- Importer representation and FSSAI license for food supplements
- Review of certificates (FSC, GMP , COA) and third-party validations
- Custom clearance coordination and compliance with FSSAI’s port inspection standards
Scope of Products Covered in FSSAI Food Registration in India
DDReg’s Experience Across Food Supplement Categories
| Category | Typical Products & Examples |
|---|---|
| Health Supplements | Vitamins, minerals, amino acids, enzymes |
| Nutraceuticals | Plant-based extracts, antioxidants, fatty acids |
| Functional Foods & Beverages | Protein bars, energy drinks, fortified juices |
| Foods for Special Dietary Use | Diabetic-friendly supplements, senior nutrition products |
| FSMP (Special Medical Purpose) | Renal nutrition, metabolic disorder formulations |
| Herbal & Ayurceutical Products | Curcumin, Ashwagandha, Green Tea Extract (within limits) |
| Probiotic & Prebiotic Products | Synbiotic sachets, gut-health capsules |
| Sports Nutrition | BCAAs, creatine, electrolyte powders |
Why Choose DDReg for FSSAI Health Supplement Registration in India?
- Scientific writing + regulatory strategy , our core strength in proprietary food dossiers
- Proven track record of FSSAI approvals across health supplement SKUs
- Expertise in both FoSCoS and pre-FoSCoS licensing regimes
- End-to-end lifecycle management: from ingredient validation to license renewal
- Dedicated India-based team with reach across CDSCO, FSSAI, and customs authorities