DDReg: Your Partner for Cosmetic Product Regulatory Compliance
The cosmetics sector in India is driven by increasing demand for personal care products, cross-border beauty trends, and evolving consumer preferences. However, bringing a doing a cosmetic product registration in the Indian market, whether manufactured locally or imported, requires thorough knowledge of the Cosmetic Rules, 2020 under the Drugs and Cosmetics Act, 1940.
At DDReg, we provide specialized Cosmetics Regulatory Services for Manufacturers and importers, covering every requirement from CDSCO cosmetic registration and labeling review to Cosmetics Import Registration in India and post-approval lifecycle support.
Understanding India's Cosmetic Regulatory Landscape
India’s cosmetic regulations are enforced by the Central Drugs Standard Control Organization (CDSCO), with clearly defined rules for the import, manufacture, labeling, and sale of cosmetic products.
Whether you're a global beauty brand entering India or an Indian manufacturer expanding your portfolio, compliance is non-negotiable. Each cosmetic product must:
- Be registered under CDSCO before being imported
- Follow BIS standards (where applicable) and avoid restricted/prohibited ingredients
- Adhere to labeling regulations including manufacturer/importer name, expiry, MRP, and batch details
- Maintain documentation for inspection and renewal as per Cosmetic Rules, 2020
DDReg’s CDSCO Cosmetic Regulatory Services in India
As your trusted regulatory partner, we offer tailored services for both Indian manufacturers and global cosmetic brands, ensuring compliance from product registration to ongoing lifecycle management.
-
Cosmetic Product Registration in India
-
Cosmetics Import Registration in India
-
CDSCO Cosmetics Manufacturers Services
-
Labeling, Claims & Ingredient Review
-
Post-Registration & Lifecycle Management
Support for Foreign Brands and Importers
DDReg supports numerous global brands with Cosmetics Import Registration in India through end-to-end project execution. Our Bi-lateral Support Model ensures:
- Local representation as Authorized Indian Agent
- Dedicated submission team for CDSCO’s SUGAM portal
- Indian market readiness review: ingredients, packaging, and label compliance
- Assistance with customs coordination and supply chain partners
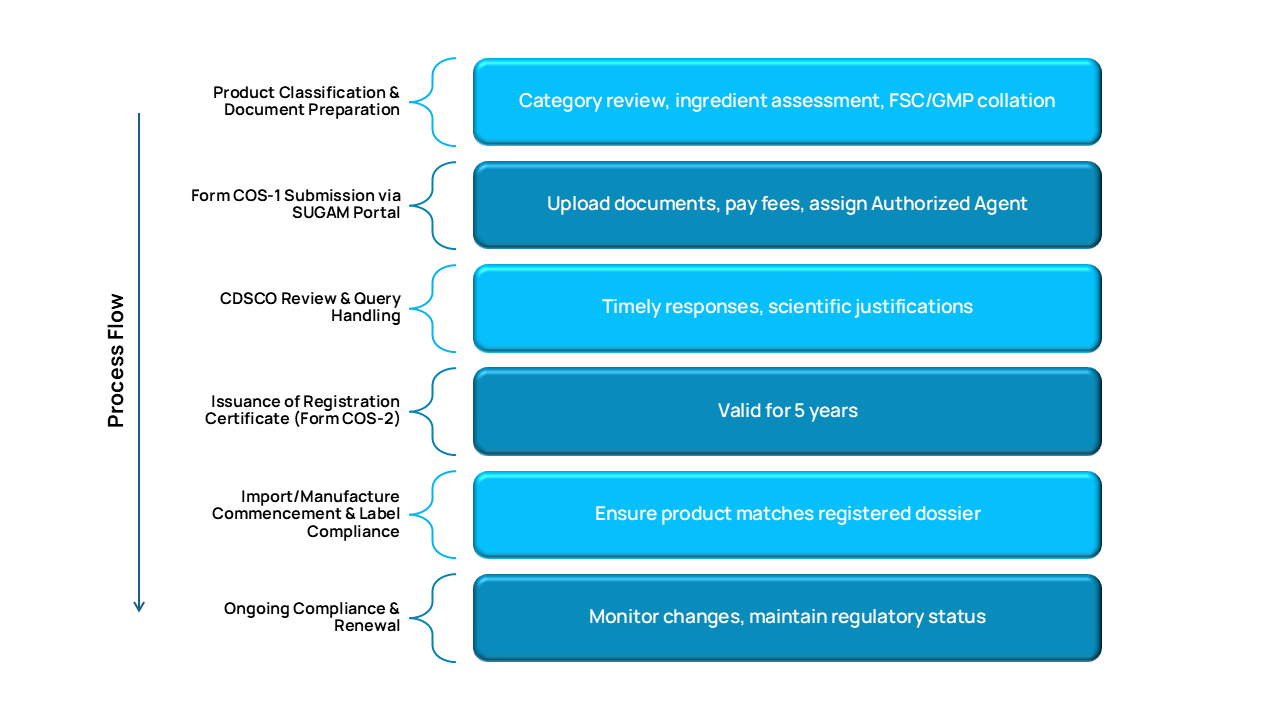
CDSCO Regulatory Pathways for Cosmetics
Key Registration Routes under CDSCO
| Pathway | Applicable To | Regulatory Form |
|---|---|---|
| Import Registration | Foreign cosmetic brands | Form COS-1 / COS-2 |
| Manufacturing License | Indian cosmetic manufacturers | Form COS-5 / COS-8 |
| Label Compliance Review | All marketed cosmetic products | CDSCO & BIS Guidance |
| Authorized Agent Support | Overseas companies without Indian base | COS-1 Filing Support |
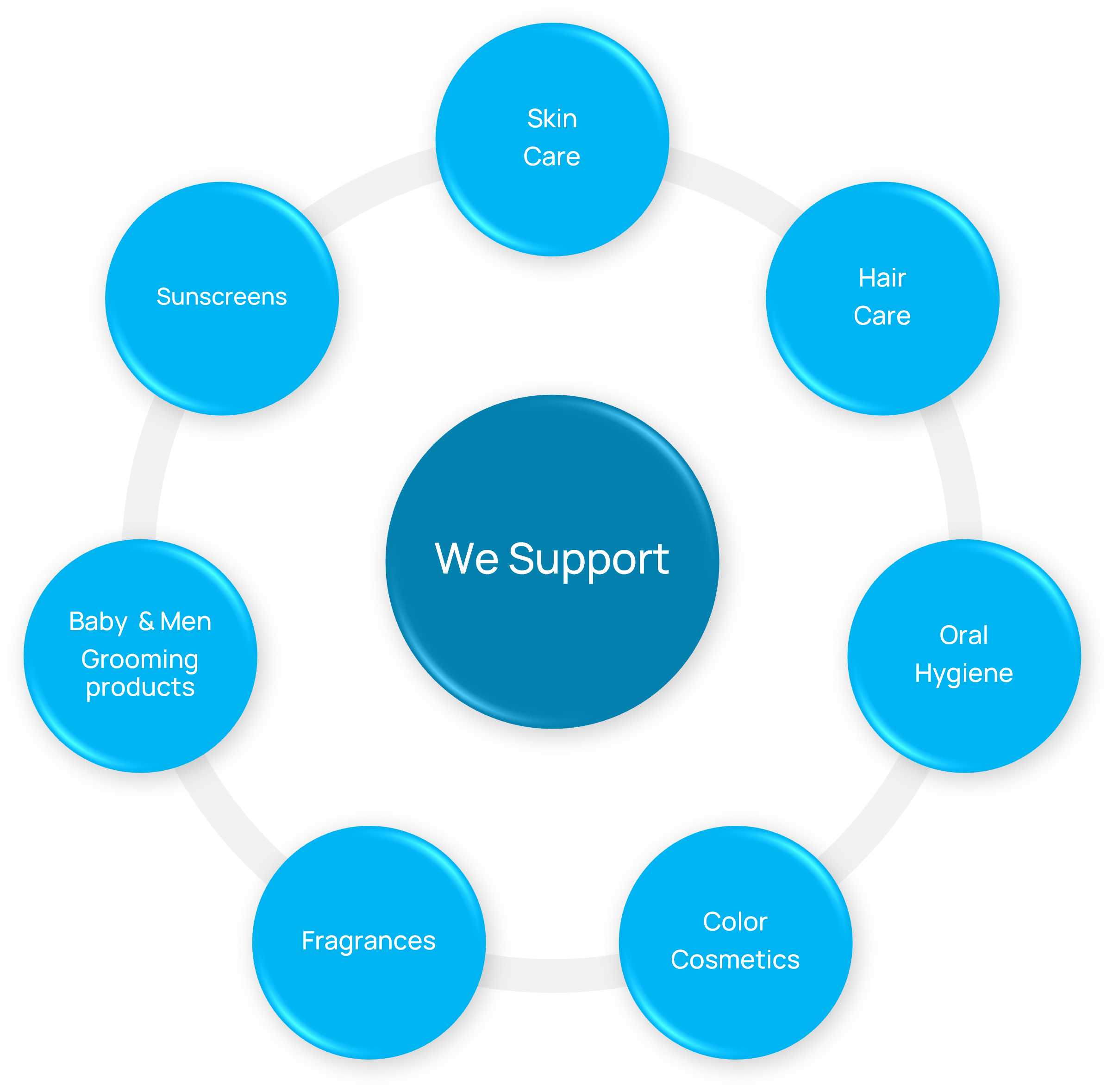
Cosmetic Product Categories We Support
Our services extend across the entire cosmetic portfolio:
How We Work: Cosmetic Product Registration Process Flow