Clinical Trial Execution, Audit, and Regulatory Submission Support
< p > India’s evolving clinical trial research landscape offers immense scientific and strategic advantages, but only if approached with regulatory foresight and executional clarity.Whether you're conducting a global clinical trial (GCT) or a local pivotal study, DDReg delivers comprehensive, regulatory-aligned clinical trial research services in India tailored to meet NDCT Rules, 2019, ICH-GCP, and CDSCO mandates.
From clinical trial submissions and preclinical data interpretation to clinical trial site management in India, our team acts as interconnectors amongst sponsors, CROs, investigators, and Indian regulatory authorities.
India’s Clinical Research Services Framework – At a Glance
- Governed by the Drugs & Clinical Trials Rules, 2019 (NDCT Rules) under CDSCO
- Mandatory regulatory approvals for new drugs, devices, BA/BE studies, and PMS
- Clinical studies must be registered on the Clinical Trials Registry – India (CTRI)
- Oversight by Subject Expert Committees (SECs) and Institutional Ethics Committees (IECs)
DDReg’s Clinical Trial Research Services in India
-
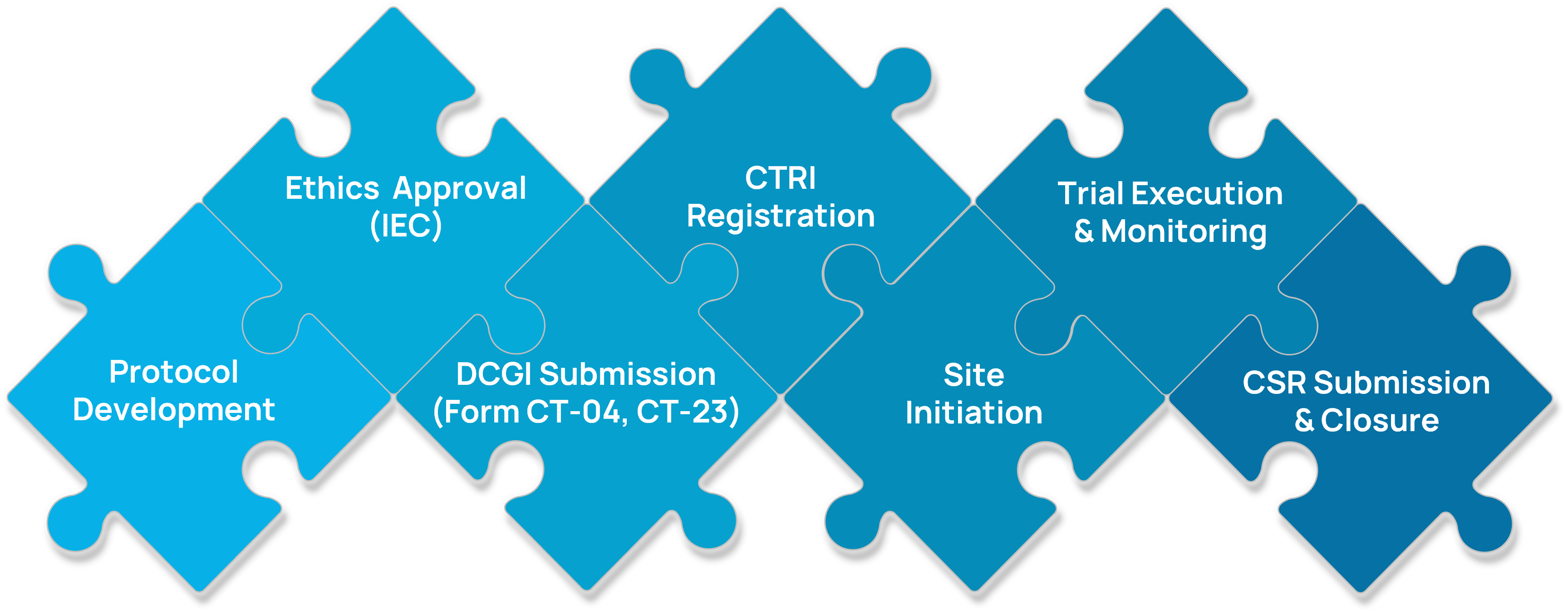
Regulatory Submissions & Strategy (GCT & Local Trials)
- Gap analysis and adaptation of global protocols to Indian regulatory norms
-
Preparation and submission of:
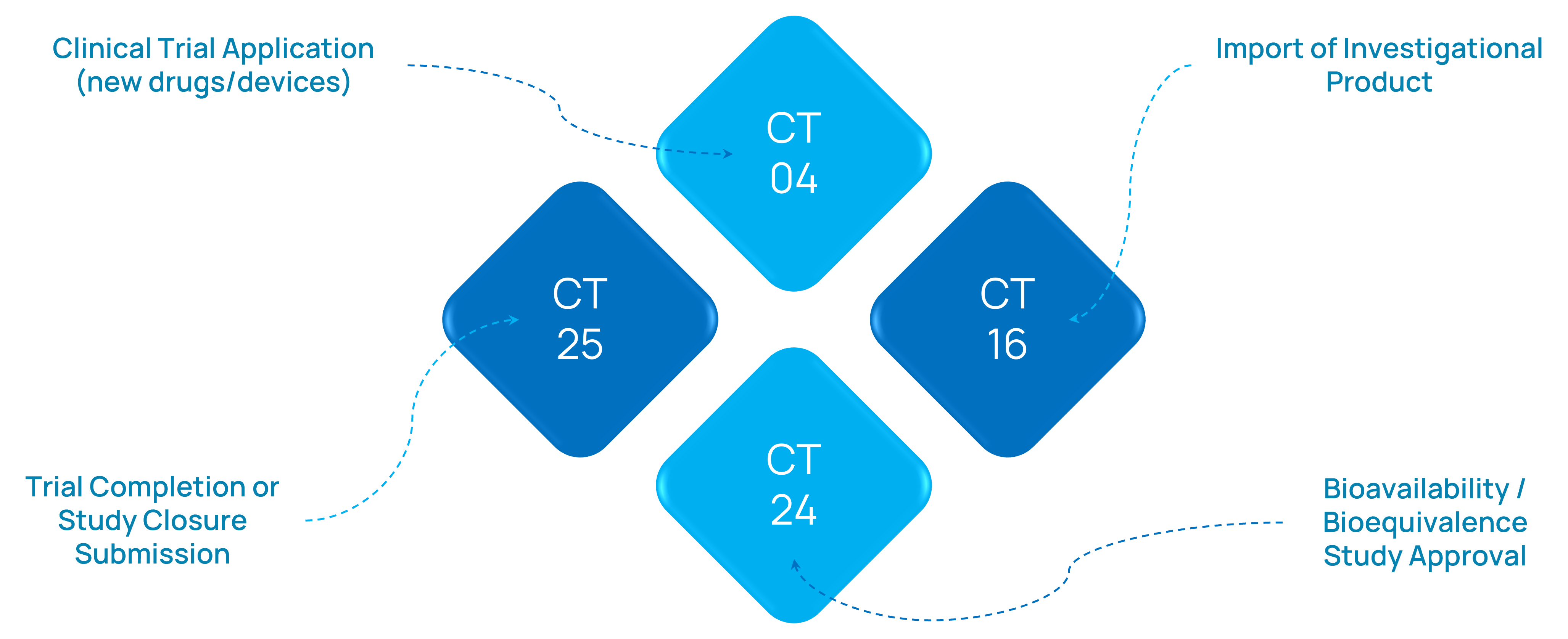
- Form CT-04 / CT-23 for trial approvals
- Form CT-16 for import of investigational drugs
- CTRI registration documentation
- End-to-end liaison with CDSCO and SECs for both global clinical trials (GCTs) and local Phase I–IV studies
-
Clinical Research Consulting India
-
Preclinical & Translational Support
Step-by-Step CTR Submission Timeline:
Full-Service Clinical Trial Operations
-
Site Start-Up & Management
-
Clinical Trial Monitoring
-
Safety Management
Study Types We Cover in Clinical Research Services
| Type | Scope |
|---|---|
| Global Clinical Trials (GCT) | Full regulatory submissions + oversight for India sites |
| Local Phase I–IV Trials | Indian studies for NDAs, SNDAs, FDCs |
| BA/BE Studies | Regulatory and operational support for BA/BE & PK studies |
| Post-Marketing Surveillance | Safety data collection, AE tracking, and reporting |
| Investigator-Initiated Trials | Regulatory review and safety support |
Sample Submission Forms We Handle
Why Choose DDReg for Clinical Research Services in India?
- Real-world experience with DCGI, CDSCO, and SEC interactions
- Audited network of clinical trial vendors, CROs, and labs across India
- Robust systems for data compliance, SAE management, and GCP documentation
- One-point solution for clinical trial services in India — strategy to closure
Country Specific Services