Commercial Entry Requirements with Expert India Authorized Agent Support
For manufacturers looking to launch pharmaceuticals, medical devices, cosmetics, nutraceuticals, or FMCG products in India, regulatory representation is mandatory. Without an office in India, you must appoint an India Authorized Agent to interface with CDSCO and other authorities, ensure end-to-end compliance, and handle all local obligations on your behalf.
For manufacturers looking to launch pharmaceuticals, medical devices, cosmetics, nutraceuticals, or FMCG products in India, regulatory representation is mandatory. Without an office in India, you must appoint an India Authorized Agent to interface with CDSCO and other authorities, ensure end-to-end compliance, and handle all local obligations on your behalf.
Our End-to-End India Authorized Agent Services
-
Our End-to-End India Authorized Agent Services
-
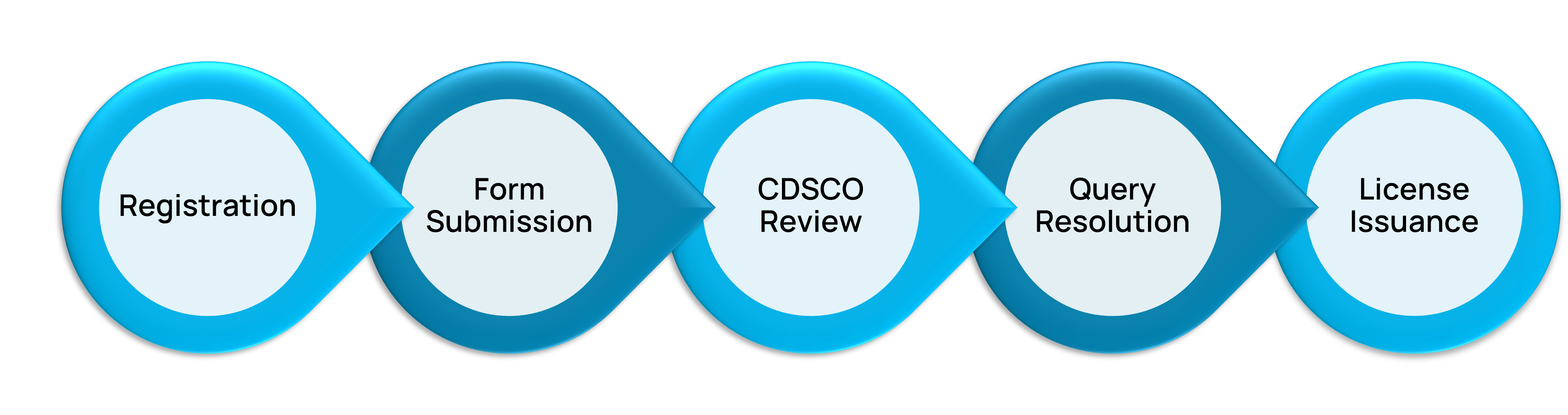
Drug Registration Authorized Agent India – SUGAM Portal Compliance
-
Wholesale & Retail Licensing Services in India
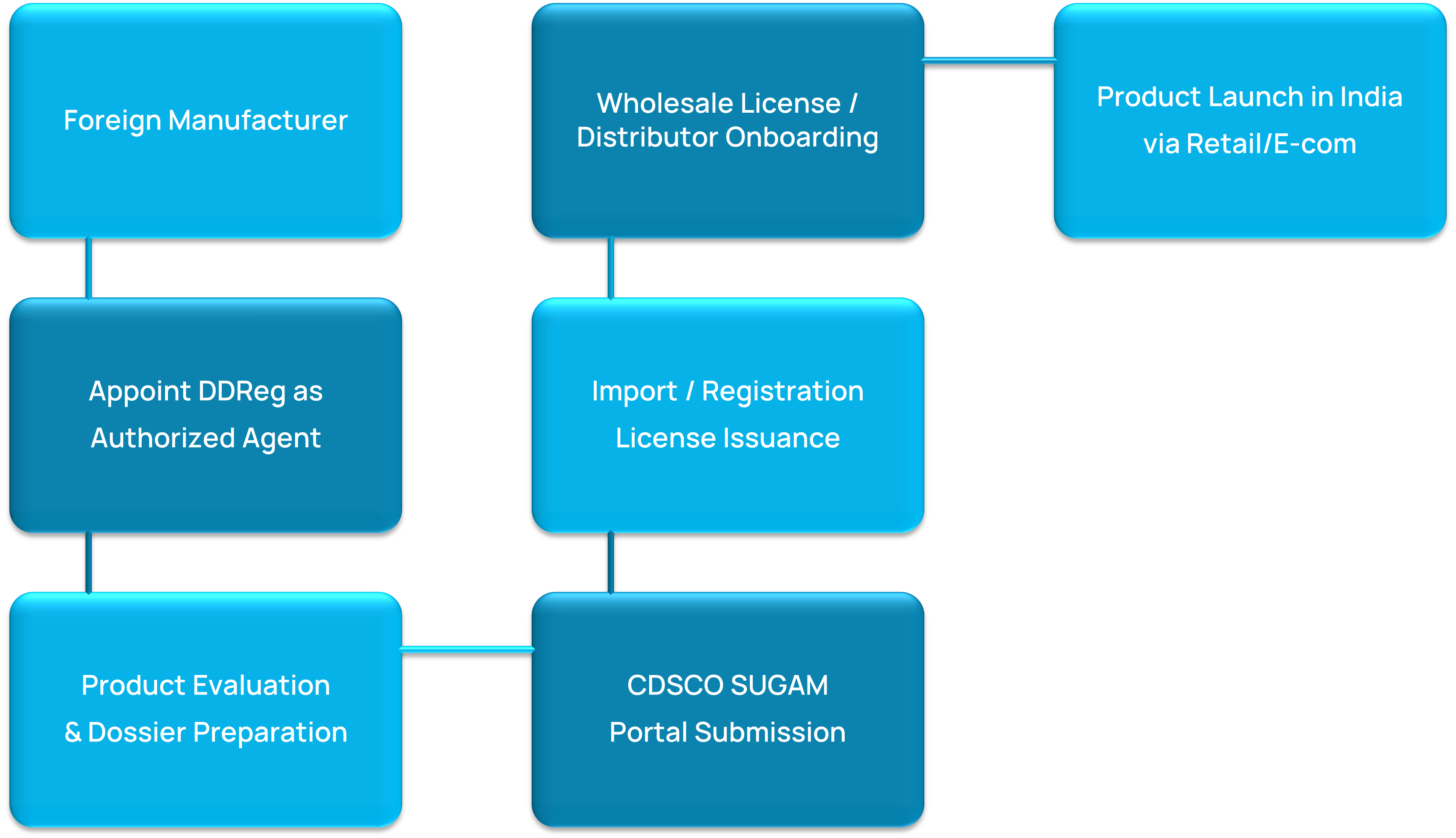
License Type Purpose Import Registration For registration of foreign manufacturing sites Import Licence License to import drugs & Medical Devices Wholesale Drug License For selling drugs to retailers, hospitals, or institutions Retail Drug License Required to sell drugs directly to consumers FMCG / Cosmetic Distribution License Applicable for fast-moving consumer goods, beauty, and wellness products We liaise with State FDA Authorities to handle site inspections, document submissions, and application follow-ups ensuring complete readiness for issuance.
-
Business Licensing & Market Readiness Enablement
Representation & Licensing Pathway for Foreign Manufacturers
Why Choose DDReg for Authorized Agent Support Services Provider?
- Registered CDSCO liaison and longstanding India Authorized Agent for multiple global firms
- Familiarity with product registration across drugs, cosmetics, medical devices, and supplements
- Local knowledge of state licensing, warehouse registrations, and product labeling norms
- One-stop compliance partner from regulatory filing to market entry